Introduction
What is Synovial Sarcoma?
Synovial sarcoma is a rare and aggressive form of soft tissue cancer that arises near joints, primarily in adolescents and young adults. Despite its name, it doesn’t originate from synovial tissues but is named due to its location and histological resemblance.
Representing about 5–10% of all soft tissue sarcomas, this cancer typically affects the arms, legs, and trunk. Due to its subtle early symptoms and deep anatomical location, it is often diagnosed late—posing a major challenge to effective treatment.
Global Disease Burden and Treatment Needs
Globally, synovial sarcoma accounts for 1–3 new cases per million people annually, with a higher prevalence in younger populations. As with many rare cancers, treatment options have historically been limited, emphasizing the need for innovation, early detection, and multimodal therapies.
Market Overview
Market Size and Forecast
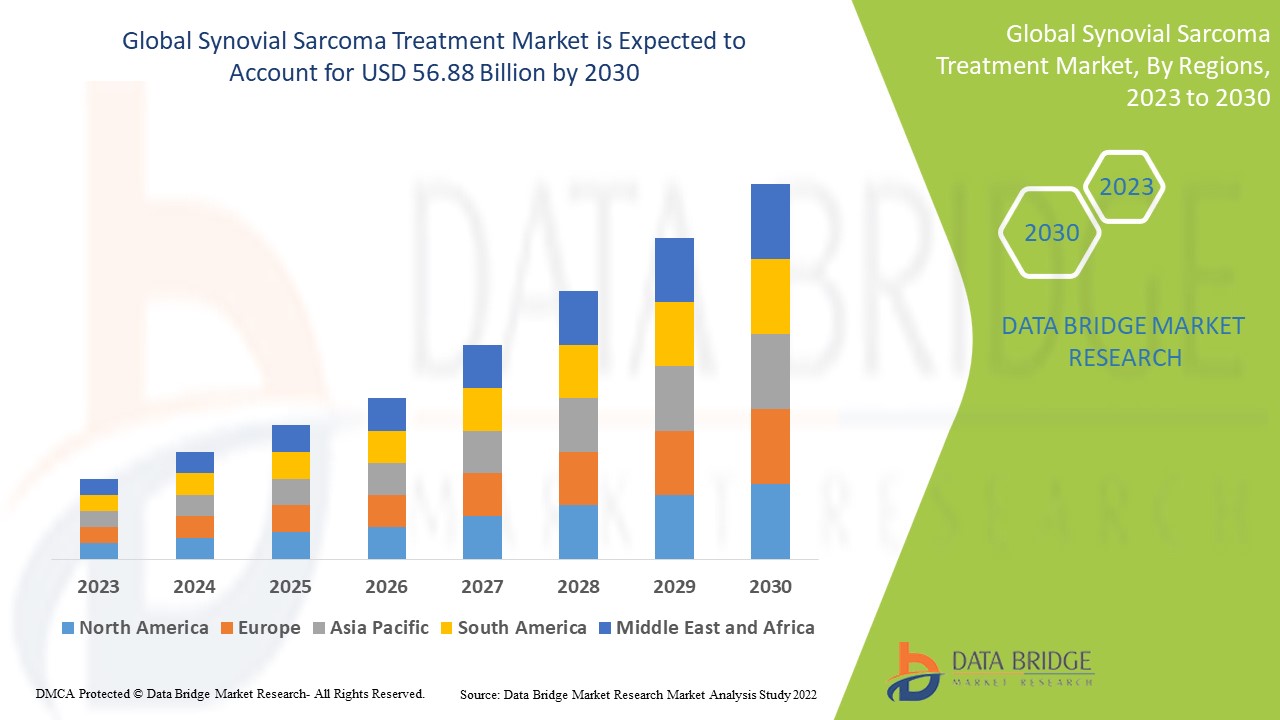
The global synovial sarcoma treatment market was valued at approximately USD 310 million in 2023 and is projected to surpass USD 540 million by 2030, growing at a CAGR of 8.5%. This growth is driven by increasing research investments, orphan drug approvals, and emerging targeted therapies.
Key Stakeholders in the Treatment Ecosystem
-
Pharmaceutical and biotech companies
-
Research institutions and cancer centers
-
Regulatory agencies
-
Patient advocacy groups
-
Health insurance providers
These entities collaborate in drug development, patient access programs, clinical trials, and awareness campaigns to improve outcomes in this niche market.
Market Segmentation
By Treatment Type
-
Surgery (primary treatment for localized tumors)
-
Radiation Therapy
-
Chemotherapy (Doxorubicin, Ifosfamide)
-
Targeted Therapy
-
Immunotherapy
-
Experimental/Investigational Drugs
By Disease Stage
-
Localized Synovial Sarcoma
-
Metastatic Synovial Sarcoma
-
Recurrent Cases
By Route of Administration
-
Oral
-
Intravenous (IV)
-
Subcutaneous
-
Inhalation (experimental)
By End-User
-
Hospitals and Cancer Centers
-
Specialty Clinics
-
Academic and Research Institutes
-
Homecare Settings (for oral/targeted therapies)
By Region
-
North America
-
Europe
-
Asia-Pacific
-
Latin America
-
Middle East & Africa
Regional Insights
North America
North America dominates the market due to high healthcare spending, strong oncology infrastructure, and active clinical trial ecosystems. The United States in particular sees consistent FDA approvals for orphan drugs and compassionate use programs.
Europe
Europe contributes significantly to rare cancer research, with countries like Germany, France, and the UK offering comprehensive sarcoma care pathways. The European Medicines Agency (EMA) supports drug development via orphan designation incentives.
Asia-Pacific
The Asia-Pacific region is witnessing growth due to improving cancer diagnostics and increasing investment in clinical oncology. However, late-stage diagnosis and uneven healthcare access still limit treatment effectiveness in many parts of the region.
Latin America
In Latin America, increasing cancer burden and awareness are creating opportunities for international collaborations. Access to innovative therapies remains limited, although governments are working on affordability and import-friendly regulations.
Middle East & Africa
This region sees the lowest diagnosis and survival rates, primarily due to limited awareness, diagnostic delays, and restricted access to high-cost treatments. However, ongoing health reforms are paving the way for specialty oncology centers.
Market Drivers
Increasing Incidence of Rare Cancers
There is growing recognition and diagnosis of rare and ultra-rare cancers like synovial sarcoma. With more genomic testing, cases are being accurately classified, leading to better-targeted treatment pathways.
Advancements in Targeted Therapy
The approval of agents like Tazemetostat and Pazopanib, as well as the research surrounding NY-ESO-1 T-cell therapy, demonstrates significant promise. These targeted therapies offer greater efficacy with fewer systemic side effects than traditional chemotherapy.
Growing Clinical Trials and Regulatory Approvals
Orphan drug designations and fast-track approvals have created a favorable regulatory environment. Many biotech startups are receiving grants and venture funding to explore novel biologics and cell-based therapies.
Market Challenges
Limited Awareness and Misdiagnosis
Due to its rarity and non-specific early symptoms, synovial sarcoma is often misdiagnosed as a benign cyst or soft tissue mass. Delayed diagnosis impacts survival rates and limits eligibility for curative surgery.
High Cost of Biologics and Targeted Drugs
Advanced therapies such as monoclonal antibodies, CAR-T cells, and immunomodulators come with high costs, often out of reach for patients in middle- and low-income countries.
Small Patient Population and Trial Limitations
The rarity of synovial sarcoma leads to limited clinical data and difficulty in conducting large-scale trials. This often results in slower drug development and reluctance from investors.
Emerging Trends
Personalized and Precision Medicine
Advancements in tumor gene profiling are enabling patient-specific treatment regimens, minimizing trial-and-error therapy, and enhancing response rates. Molecular diagnostics are increasingly used in treatment decision-making.
Immunotherapy and Checkpoint Inhibitors
While still under investigation, immune checkpoint inhibitors (like PD-1/PD-L1 blockers) are being tested as potential game changers in the treatment of synovial sarcoma, especially in refractory or metastatic cases.
Role of Orphan Drug Status and Fast-Track Designations
Governments across the U.S., EU, and Japan are offering incentives like tax breaks, reduced clinical trial requirements, and market exclusivity to accelerate drug development in rare cancers.
Competitive Landscape
Leading Players
-
Adaptimmune Therapeutics – working on NY-ESO-1 TCR therapy
-
Eli Lilly and Company
-
Epizyme, Inc. – developer of Tazemetostat
-
Bristol Myers Squibb
-
Pfizer Inc. – with targeted cancer drug portfolio
-
Novartis AG
-
Merck & Co., Inc.
These companies focus on targeted oncology, immunotherapy pipelines, and collaborations with academic cancer centers for Phase I–III studies.
Strategic Initiatives
-
Expansion of orphan oncology portfolios
-
Co-development deals with gene therapy startups
-
Licensing agreements with academic institutions
-
Launch of patient access programs and compassionate use pathways
SWOT Analysis
| Strengths | Weaknesses |
|---|---|
| Advances in personalized treatment options | Small patient population limits ROI for pharma |
| Supportive regulatory environment for orphan drugs | High treatment cost and access inequality |
| Strong collaboration between pharma and academia | Risk of relapse and limited curative options |
| Opportunities | Threats |
|---|---|
| Increasing clinical trials and pipeline development | Regulatory delays in developing regions |
| Entry of biosimilars and cost-effective therapies | Discontinuation of trials due to poor recruitment |
| Immunotherapy and T-cell based treatment innovation | Low awareness among primary care physicians |
Future Outlook and Opportunities
The future of the synovial sarcoma treatment market lies in the integration of multi-modal therapies, combining surgery with targeted drugs and immunotherapy. Breakthroughs in gene editing, cell therapy, and AI-guided treatment protocols hold transformative potential.
Key Growth Opportunities
-
Expansion into Asia-Pacific and Latin America with cost-effective therapies
-
Launch of AI-driven diagnostic platforms to reduce misdiagnosis
-
Increased funding from cancer foundations for rare disease research
-
Development of oral targeted drugs to simplify treatment administration
As personalized oncology care becomes mainstream, patients with synovial sarcoma may soon benefit from tailored treatment pathways, improving both survival and quality of life.
Conclusion
The synovial sarcoma treatment market, while niche, is undergoing a quiet revolution. Increased awareness, advances in biotechnology, and regulatory support are accelerating the discovery of innovative therapies for this rare cancer.
Though challenges remain—especially around access, awareness, and high treatment costs—collaborative global efforts are reshaping the landscape. The market is poised to grow rapidly as more targeted drugs, cell therapies, and diagnostic tools reach patients worldwide.
In this rare but impactful oncology segment, every medical breakthrough doesn’t just add value—it saves lives.
Get More Details : https://www.databridgemarketresearch.com/reports/global-synovial-sarcoma-treatment-market
Get More Reports :
https://www.databridgemarketresearch.com/reports/us-dental-insurance-market
https://www.databridgemarketresearch.com/reports/global-melasma-treatment-market
https://www.databridgemarketresearch.com/reports/global-liquid-heat-exchanger-system-market




0 Comments